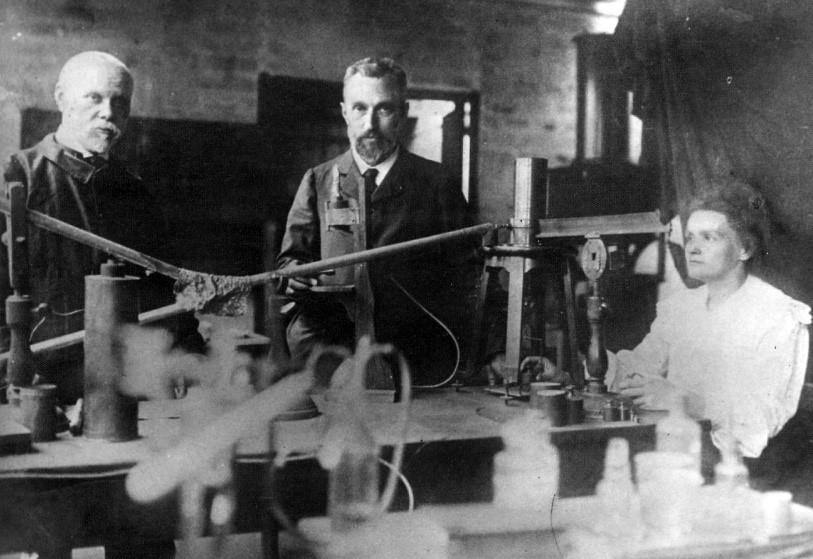
Separation of Radium by Marie and Pierre Curie

Sorbonne-bred physicist Pierre Curie had been noodling with crystals and magnetism since the early 1880s. He was a professor at the School of Physics in Paris when one of his students, Marie Sklodowska, caught his eye. A colleague of the Curies, Henri Becquerel, paved the way for their groundbreaking research with his discovery of spontaneous radioactivity in 1896. Becquerel's presentation to the Academy of Sciences aroused little interest. Dragging Pierre away from his crystals, Marie got the ball rolling on what would be the central pillar of their life's work.
Following on Becquerel, the Curies succeeded in isolating element 84, Polonium (named for Poland, the country of Marie's birth), and then element 88, Padium in 1898. It was Marie, in particular, who devised a method for separating radium from its radioactive residues, making possible the closer study of its therapeutic properties.
Radium is an element of atomic weight 226, the highest term in the alkaline earth series. It is a metal having many analogies with barium and it is also a “radioactive substance”, i.e., a substance that suffers a spontaneous disintegration accompanied by the emission of radiation. This radioactive property confers on radium a special importance for scientific purposes or for medical use, and is also the cause of the extreme rarity of the element. Though radium is only one of numerous radioactive substances, being neither the most radioactive nor the most abundant, its rate of decay and the nature of the products of its disintegration have proved particularly favourable in the applications of radioactivity, and make it the most important of radioelements.
Radioactive substances emit three kinds of rays known as α-, β- and γ-rays. The α-rays are helium nuclei carrying each a positive charge equal to double that of the elementary charge; they are expelled from the nuclei of the radioactive atoms with a great velocity (about 1.5x109 to 2.3x109 cm/s). The β-rays are electrons of various velocities which may approach the velocity of light. The γ-rays constitute an electromagnetic radiation of the same kind as light or X-rays, but their wave-length is generally much smaller and may be as short as 0.01 Å. While the emission of some radioelements consists almost entirely of α-rays whose penetrating power is very small, other radioelements emit β- and γ-rays which are able to penetrate a considerable thickness of matter.
The curie is the international unit of measurement for radioactivity. Although originally defined as the radioactivity of 1 gram of pure radium, it is now specified as 3.7x1010 atomic disintegrations per second, or 37 gigabecquerels.




REFERENCES
Encyclopædia Britannica. Available in: https://www.britannica.com/topic/Marie-Curie-and-Irene-Curie-on-radium-1983710. Access in: 17/09/2018.
Wired. Available in: https://www.wired.com/2009/12/1221curies-discover-radium/. Access in: 17/09/2018.


























0 comments
Sign in or create a free account