Planck's quantum hypothesis
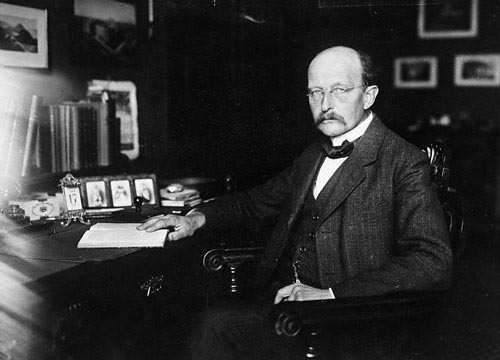
Max Planck (1858-1947) is a German scientist who set out to describe the spectral distribution of the blackbody so that there was a mathematical formulation that fit with what was measured experimentally, as opposed to the divergence that existed for classical theory (ultraviolet catastrophe).
The quantum hypothesis postulates that electromagnetic radiation can only be emitted and absorbed in discrete bundles called quanta. This work that provided the foundation for his quantum theory. Planck found that the vibrational energy of atoms in a solid is not continuous but has only discrete (distinct) values. Light energy is determined by its frequency of vibration, f. Energy, E, is described by the equation:
E = nhf
where n is an integer and h is Planck's constant, equal to 6.626068×10−34 J.s.
Planck published his theory “On the Law of Distribution of Energy in the Normal Spectrum” in the German journal Annalen der Physikm, stating:
“Moreover, it is necessary to interpret UN ‘the total energy of a blackbody radiator’ not as a continuous, infinitely divisible quantity, but as a discrete quantity composed of an integral number of finite equal parts.”
Planck’s concept of energy quanta, in other words, conflicted fundamentally with all past physical theory. He made many contributions to theoretical physics, but his fame rests primarily on his role as originator of the quantum theory. This theory revolutionized our understanding of atomic and subatomic processes.
Planck's article transcribed in English

REFERENCES
EISBERG; RESNICK. Física Quântica. 23ª Ed. Elsevier. p. 17-20, 32-38, 42.
Encyclopædia Britannica. Available in: https://www.britannica.com/biography/Max-Planck. Access in: 24/08/2018.




















0 comments
Sign in or create a free account