Discovery of Atomic Nucleus and Rutherford model of the atom

Rutherford atomic model, also called nuclear atom or planetary model of the atom, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. Rutherford directed the famous Geiger–Marsden experiment in 1909 which suggested, upon Rutherford's 1911 analysis, that J. J. Thomson's plum pudding model of the atom was incorrect. Rutherford's new model for the atom, based on the experimental results, contained the new features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom and with this central volume also containing the bulk of the atomic mass of the atom. This region would be known as the "nucleus" of the atom.
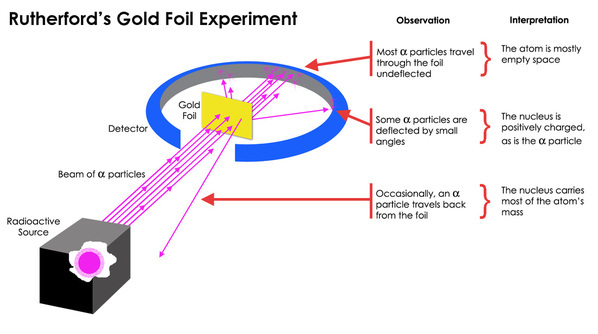
Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. If Thomson was correct, the beam would go straight through the gold foil. Most of the beams went through the foil, but a few were deflected by the foil and hit a spot on a screen placed off to one side. One in 20,000 alpha particles had been deflected 45º or more. Rutherford asked why so many alpha particles passed through the gold foil while a few were deflected so greatly. The only explanation is that there would be a mass agglomerate that would have a positive charge and repel the alpha particle, causing the scattering.
The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.

Rutherford's article on the scattering of alpha particles


REFERENCES
TIPLER, P. A. e LLEWELLYN, R. A. Modern Physics. 6ª ed. Freeman and Company, 2012. p. 156-162.
Encyclopædia Britannica. Available in: https://www.britannica.com/science/Rutherford-atomic-model. Access in: 24/08/2018.
Encyclopædia Britannica. Available in: https://www.britannica.com/science/atom/Discovery-of-radioactivity#ref496652. Access in: 03/09/2018.




















0 comments
Sign in or create a free account